Myocardial infarction
| Myocardial infarction | |
|---|---|
| Classification and external resources | |
 Diagram of a myocardial infarction (2) of the tip of the anterior wall of the heart (an apical infarct) after occlusion (1) of a branch of the left coronary artery (LCA, right coronary artery = RCA). |
|
| ICD-10 | I21.-I22. |
| ICD-9 | 410 |
| DiseasesDB | 8664 |
| MedlinePlus | 000195 |
| eMedicine | med/1567 emerg/327 ped/2520 |
| MeSH | D009203 |
Myocardial infarction (MI) or acute myocardial infarction (AMI), commonly known as a heart attack, is the interruption of blood supply to part of the heart, causing heart cells to die. This is most commonly due to occlusion (blockage) of a coronary artery following the rupture of a vulnerable atherosclerotic plaque, which is an unstable collection of lipids (fatty acids) and white blood cells (especially macrophages) in the wall of an artery. The resulting ischemia (restriction in blood supply) and oxygen shortage, if left untreated for a sufficient period of time, can cause damage or death (infarction) of heart muscle tissue (myocardium).
Classical symptoms of acute myocardial infarction include sudden chest pain (typically radiating to the left arm or left side of the neck), shortness of breath, nausea, vomiting, palpitations, sweating, and anxiety (often described as a sense of impending doom)[1]. Women may experience fewer typical symptoms than men, most commonly shortness of breath, weakness, a feeling of indigestion, and fatigue.[2] Approximately one quarter of all myocardial infarctions are "silent", without chest pain or other symptoms.
Among the diagnostic tests available to detect heart muscle damage are an electrocardiogram (ECG), chest X-ray, and various blood tests. The most often used markers are the creatine kinase-MB (CK-MB) fraction and the troponin levels. Immediate treatment for suspected acute myocardial infarction includes oxygen, aspirin, and sublingual nitroglycerin. If further pain relief is needed, morphine sulfate should be avoided. Recent research has shown that morphine actually increases mortality in acute coronary syndromes. The CRUSADE Trial and Registry in North Carolina, at Duke University has demonstrated that of all patients admitted to a hospital with Acute Coronary Syndrome (chest pain) in North Carolina, the ones that received morphine had a 40% increase in mortality. Morphine has been removed from the American Heart Association Guidelines for ACS since then.[3]
A 2009 review about the use of high flow oxygen for treating myocardial infarction found high flow oxygen administration increased mortality and infarct size, calling into question the recommendation for its routine use.[4] Most cases of STEMI are treated with thrombolysis or percutaneous coronary intervention (PCI). NSTEMI should be managed with medication, although PCI is often performed during hospital admission. In people who have multiple blockages and who are relatively stable, or in a few emergency cases, bypass surgery maybe an option.
Heart attacks are the leading cause of death for both men and women worldwide.[5] Important risk factors are previous cardiovascular disease, older age, tobacco smoking, high blood levels of certain lipids (triglycerides, low-density lipoprotein) and low levels of high density lipoprotein (HDL), diabetes, high blood pressure, obesity, chronic kidney disease, heart failure, excessive alcohol consumption, the abuse of certain drugs (such as cocaine and methamphetamine), and chronic high stress levels.[6][7]
Contents |
Classification
There are two basic types of acute myocardial infarction:
- Transmural: associated with atherosclerosis involving major coronary artery. It can be subclassified into anterior, posterior, or inferior. Transmural infarcts extend through the whole thickness of the heart muscle and are usually a result of complete occlusion of the area's blood supply.[8]
- Subendocardial: involving a small area in the subendocardial wall of the left ventricle, ventricular septum, or papillary muscles. Subendocardial infarcts are thought to be a result of locally decreased blood supply, possibly from a narrowing of the coronary arteries. The subendocardial area is farthest from the heart's blood supply and is more susceptible to this type of pathology.[8]
Clinically, a myocardial infarction can be further subclassified into a ST elevation MI (STEMI) versus a non-ST elevation MI (non-STEMI) based on ECG changes.[9]
The phrase "heart attack" is sometimes used incorrectly to describe sudden cardiac death, which may or may not be the result of acute myocardial infarction. A heart attack is different from, but can be the cause of cardiac arrest, which is the stopping of the heartbeat, and cardiac arrhythmia, an abnormal heartbeat. It is also distinct from heart failure, in which the pumping action of the heart is impaired; severe myocardial infarction may lead to heart failure, but not necessarily.
A 2007 consensus document classifies myocardial infarction into five main types:[10]
- Type 1 - Spontaneous myocardial infarction related to ischaemia due to a primary coronary event such as plaque erosion and/or rupture, fissuring, or dissection
- Type 2 - Myocardial infarction secondary to ischaemia due to either increased oxygen demand or decreased supply, e.g. coronary artery spasm, coronary embolism, anaemia, arrhythmias, hypertension, or hypotension
- Type 3 - Sudden unexpected cardiac death, including cardiac arrest, often with symptoms suggestive of myocardial ischaemia, accompanied by presumably new ST elevation, or new LBBB, or evidence of fresh thrombus in a coronary artery by angiography and/or at autopsy, but death occurring before blood samples could be obtained, or at a time before the appearance of cardiac biomarkers in the blood
- Type 4 - Associated with coronary angioplasty or stents:
- Type 4a - Myocardial infarction associated with PCI
- Type 4b - Myocardial infarction associated with stent thrombosis as documented by angiography or at autopsy
- Type 5 - Myocardial infarction associated with CABG
Signs and symptoms

The onset of symptoms in myocardial infarction (MI) is usually gradual, over several minutes, and rarely instantaneous.[11] Chest pain is the most common symptom of acute myocardial infarction and is often described as a sensation of tightness, pressure, or squeezing. Chest pain due to ischemia (a lack of blood and hence oxygen supply) of the heart muscle is termed angina pectoris. Pain radiates most often to the left arm, but may also radiate to the lower jaw, neck, right arm, back, and epigastrium, where it may mimic heartburn. Levine's sign, in which the patient localizes the chest pain by clenching their fist over the sternum, has classically been thought to be predictive of cardiac chest pain, although a prospective observational study showed that it had a poor positive predictive value.[12]
Shortness of breath (dyspnea) occurs when the damage to the heart limits the output of the left ventricle, causing left ventricular failure and consequent pulmonary edema. Other symptoms include diaphoresis (an excessive form of sweating)[1], weakness, light-headedness, nausea, vomiting, and palpitations. These symptoms are likely induced by a massive surge of catecholamines from the sympathetic nervous system[13] which occurs in response to pain and the hemodynamic abnormalities that result from cardiac dysfunction. Loss of consciousness (due to inadequate cerebral perfusion and cardiogenic shock) and even sudden death (frequently due to the development of ventricular fibrillation) can occur in myocardial infarctions.
Women and older patients report atypical symptoms more frequently than their male and younger counterparts.[14] Women also report more numerous symptoms compared with men (2.6 on average vs 1.8 symptoms in men).[14] The most common symptoms of MI in women include dyspnea, weakness, and fatigue. Fatigue, sleep disturbances, and dyspnea have been reported as frequently occurring symptoms which may manifest as long as one month before the actual clinically manifested ischemic event. In women, chest pain may be less predictive of coronary ischemia than in men.[15]
Approximately half of all MI patients have experienced warning symptoms such as chest pain prior to the infarction.[16]
Approximately one fourth of all myocardial infarctions are silent, without chest pain or other symptoms.[17] These cases can be discovered later on electrocardiograms, using blood enzyme tests or at autopsy without a prior history of related complaints. A silent course is more common in the elderly, in patients with diabetes mellitus[18] and after heart transplantation, probably because the donor heart is not connected to nerves of the host.[19] In diabetics, differences in pain threshold, autonomic neuropathy, and psychological factors have been cited as possible explanations for the lack of symptoms.[18]
Any group of symptoms compatible with a sudden interruption of the blood flow to the heart are called an acute coronary syndrome.[20]
The differential diagnosis includes other catastrophic causes of chest pain, such as pulmonary embolism, aortic dissection, pericardial effusion causing cardiac tamponade, tension pneumothorax, and esophageal rupture. Other non-catastrophic differentials include gastroesophageal reflux and Tietze's syndrome.[21]
Causes
Heart attack rates are higher in association with intense exertion, be it psychological stress or physical exertion, especially if the exertion is more intense than the individual usually performs.[22] Quantitatively, the period of intense exercise and subsequent recovery is associated with about a 6-fold higher myocardial infarction rate (compared with other more relaxed time frames) for people who are physically very fit.[22] For those in poor physical condition, the rate differential is over 35-fold higher.[22] One observed mechanism for this phenomenon is the increased arterial pulse pressure stretching and relaxation of arteries with each heart beat which, as has been observed with intravascular ultrasound, increases mechanical "shear stress" on atheromas and the likelihood of plaque rupture.[22]
Acute severe infection, such as pneumonia, can trigger myocardial infarction. A more controversial link is that between Chlamydophila pneumoniae infection and atherosclerosis.[23] While this intracellular organism has been demonstrated in atherosclerotic plaques, evidence is inconclusive as to whether it can be considered a causative factor.[23] Treatment with antibiotics in patients with proven atherosclerosis has not demonstrated a decreased risk of heart attacks or other coronary vascular diseases.[24]
There is an association of an increased incidence of a heart attack in the morning hours, more specifically around 9 a.m.[25][26][27]. Some investigators have noticed that the ability of platelets to aggregate varies according to a circadian rhythm, although they have not proven causation.[28] Some investigators theorize that this increased incidence may be related to the circadian variation in cortisol production affecting the concentrations of various cytokines and other mediators of inflammation.[29]
Risk factors
Risk factors for atherosclerosis are generally risk factors for myocardial infarction:
- Diabetes (with or without insulin resistance) - the single most important risk factor for ischaemic heart disease (IHD)
- Tobacco smoking
- Hypercholesterolemia (more accurately hyperlipoproteinemia, especially high low density lipoprotein and low high density lipoprotein)
- Low HDL
- High Triglycerides
- High blood pressure
- Family history of ischaemic heart disease (IHD)
- Obesity[30] (defined by a body mass index of more than 30 kg/m², or alternatively by waist circumference or waist-hip ratio).
- Age: Men acquire an independent risk factor at age 45, Women acquire an independent risk factor at age 55; in addition individuals acquire another independent risk factor if they have a first-degree male relative (brother, father) who suffered a coronary vascular event at or before age 55. Another independent risk factor is acquired if one has a first-degree female relative (mother, sister) who suffered a coronary vascular event at age 65 or younger.
- Hyperhomocysteinemia (high homocysteine, a toxic blood amino acid that is elevated when intakes of vitamins B2, B6, B12 and folic acid are insufficient)
- Stress (occupations with high stress index are known to have susceptibility for atherosclerosis)
- Alcohol Studies show that prolonged exposure to high quantities of alcohol can increase the risk of heart attack
- Males are more at risk than females.[22]
Many of these risk factors are modifiable, so many heart attacks can be prevented by maintaining a healthier lifestyle. Physical activity, for example, is associated with a lower risk profile.[31] Non-modifiable risk factors include age, sex, and family history of an early heart attack (before the age of 60), which is thought of as reflecting a genetic predisposition.[22]
Socioeconomic factors such as a shorter education and lower income (particularly in women), and unmarried cohabitation may also contribute to the risk of MI.[32] To understand epidemiological study results, it's important to note that many factors associated with MI mediate their risk via other factors. For example, the effect of education is partially based on its effect on income and marital status.[32]
Women who use combined oral contraceptive pills have a modestly increased risk of myocardial infarction, especially in the presence of other risk factors, such as smoking.[33]
Inflammation is known to be an important step in the process of atherosclerotic plaque formation.[34] C-reactive protein (CRP) is a sensitive but non-specific marker for inflammation. Elevated CRP blood levels, especially measured with high sensitivity assays, can predict the risk of MI, as well as stroke and development of diabetes.[34] Moreover, some drugs for MI might also reduce CRP levels.[34] The use of high sensitivity CRP assays as a means of screening the general population is advised against, but it may be used optionally at the physician's discretion, in patients who already present with other risk factors or known coronary artery disease.[35] Whether CRP plays a direct role in atherosclerosis remains uncertain.[34]
Inflammation in periodontal disease may be linked coronary heart disease, and since periodontitis is very common, this could have great consequences for public health.[36] Serological studies measuring antibody levels against typical periodontitis-causing bacteria found that such antibodies were more present in subjects with coronary heart disease.[37] Periodontitis tends to increase blood levels of CRP, fibrinogen and cytokines;[38] thus, periodontitis may mediate its effect on MI risk via other risk factors.[39] Preclinical research suggests that periodontal bacteria can promote aggregation of platelets and promote the formation of foam cells.[40][41] A role for specific periodontal bacteria has been suggested but remains to be established.[42] There is some evidence that influenza may trigger a acute myocardial infarction.[43]
Baldness, hair greying, a diagonal earlobe crease (Frank's sign[44]) and possibly other skin features have been suggested as independent risk factors for MI.[45] Their role remains controversial; a common denominator of these signs and the risk of MI is supposed, possibly genetic.[46]
Calcium deposition is another part of atherosclerotic plaque formation. Calcium deposits in the coronary arteries can be detected with CT scans. Several studies have shown that coronary calcium can provide predictive information beyond that of classical risk factors.[47][48][49]
The European Society of Cardiology and the European Association for Cardiovascular Prevention and Rehabilitation have developed an interactive tool for prediction and managing the risk of heart attack and stroke in Europe. HeartScore is aimed at supporting clinicians in optimising individual cardiovascular risk reduction. The Heartscore Programme is available in 12 languages and offers web based or PC version.[50]
Pathophysiology

Acute myocardial infarction refers to two subtypes of acute coronary syndrome, namely non-ST-elevated myocardial infarction and ST-elevated myocardial infarction, which are most frequently (but not always) a manifestation of coronary artery disease.[9] The most common triggering event is the disruption of an atherosclerotic plaque in an epicardial coronary artery, which leads to a clotting cascade, sometimes resulting in total occlusion of the artery.[51][52] Atherosclerosis is the gradual buildup of cholesterol and fibrous tissue in plaques in the wall of arteries (in this case, the coronary arteries), typically over decades.[53] Blood stream column irregularities visible on angiography reflect artery lumen narrowing as a result of decades of advancing atherosclerosis.[54] Plaques can become unstable, rupture, and additionally promote a thrombus (blood clot) that occludes the artery; this can occur in minutes. When a severe enough plaque rupture occurs in the coronary vasculature, it leads to myocardial infarction (necrosis of downstream myocardium).[51][52]
If impaired blood flow to the heart lasts long enough, it triggers a process called the ischemic cascade; the heart cells in the territory of the occluded coronary artery die (chiefly through necrosis) and do not grow back. A collagen scar forms in its place. Recent studies indicate that another form of cell death called apoptosis also plays a role in the process of tissue damage subsequent to myocardial infarction.[55] As a result, the patient's heart will be permanently damaged. This Myocardial scarring also puts the patient at risk for potentially life threatening arrhythmias, and may result in the formation of a ventricular aneurysm that can rupture with catastrophic consequences.
Injured heart tissue conducts electrical impulses more slowly than normal heart tissue. The difference in conduction velocity between injured and uninjured tissue can trigger re-entry or a feedback loop that is believed to be the cause of many lethal arrhythmias. The most serious of these arrhythmias is ventricular fibrillation (V-Fib/VF), an extremely fast and chaotic heart rhythm that is the leading cause of sudden cardiac death. Another life threatening arrhythmia is ventricular tachycardia (V-Tach/VT), which may or may not cause sudden cardiac death. However, ventricular tachycardia usually results in rapid heart rates that prevent the heart from pumping blood effectively. Cardiac output and blood pressure may fall to dangerous levels, which can lead to further coronary ischemia and extension of the infarct.
The cardiac defibrillator is a device that was specifically designed to terminate these potentially fatal arrhythmias. The device works by delivering an electrical shock to the patient in order to depolarize a critical mass of the heart muscle, in effect "rebooting" the heart. This therapy is time dependent, and the odds of successful defibrillation decline rapidly after the onset of cardiopulmonary arrest.
Diagnosis
The diagnosis of myocardial infarction is made by integrating the history of the presenting illness and physical examination with electrocardiogram findings and cardiac markers (blood tests for heart muscle cell damage).[1][56] A coronary angiogram allows visualization of narrowings or obstructions on the heart vessels, and therapeutic measures can follow immediately. At autopsy, a pathologist can diagnose a myocardial infarction based on anatomopathological findings.
A chest radiograph and routine blood tests may indicate complications or precipitating causes and are often performed upon arrival to an emergency department. New regional wall motion abnormalities on an echocardiogram are also suggestive of a myocardial infarction. Echo may be performed in equivocal cases by the on-call cardiologist.[57] In stable patients whose symptoms have resolved by the time of evaluation, Technetium (99mTc) sestamibi (i.e. a "MIBI scan") or thallium-201 chloride can be used in nuclear medicine to visualize areas of reduced blood flow in conjunction with physiologic or pharmocologic stress.[57][58] Thallium may also be used to determine viability of tissue, distinguishing whether non-functional myocardium is actually dead or merely in a state of hibernation or of being stunned.[59]
Diagnostic criteria
WHO criteria[60] formulated in 1979 have classically been used to diagnose MI; a patient is diagnosed with myocardial infarction if two (probable) or three (definite) of the following criteria are satisfied:
- Clinical history of ischaemic type chest pain lasting for more than 20 minutes
- Changes in serial ECG tracings
- Rise and fall of serum cardiac biomarkers such as creatine kinase-MB fraction and troponin
The WHO criteria were refined in 2000 to give more prominence to cardiac biomarkers.[61] According to the new guidelines, a cardiac troponin rise accompanied by either typical symptoms, pathological Q waves, ST elevation or depression or coronary intervention are diagnostic of MI.
Physical examination
The general appearance of patients may vary according to the experienced symptoms; the patient may be comfortable, or restless and in severe distress with an increased respiratory rate. A cool and pale skin is common and points to vasoconstriction. Some patients have low-grade fever (38–39 °C). Blood pressure may be elevated or decreased, and the pulse can become irregular.[62][63]
If heart failure ensues, elevated jugular venous pressure and hepatojugular reflux, or swelling of the legs due to peripheral edema may be found on inspection. Rarely, a cardiac bulge with a pace different from the pulse rhythm can be felt on precordial examination. Various abnormalities can be found on auscultation, such as a third and fourth heart sound, systolic murmurs, paradoxical splitting of the second heart sound, a pericardial friction rub and rales over the lung.[62][64]
Electrocardiogram

The primary purpose of the electrocardiogram is to detect ischemia or acute coronary injury in broad, symptomatic emergency department populations. A serial ECG may be used to follow rapid changes in time. The standard 12 lead ECG does not directly examine the right ventricle, and is relatively poor at examining the posterior basal and lateral walls of the left ventricle. In particular, acute myocardial infarction in the distribution of the circumflex artery is likely to produce a nondiagnostic ECG.[65] The use of additional ECG leads like right-sided leads V3R and V4R and posterior leads V7, V8, and V9 may improve sensitivity for right ventricular and posterior myocardial infarction.
The 12 lead ECG is used to classify patients into one of three groups:[66]
- those with ST segment elevation or new bundle branch block (suspicious for acute injury and a possible candidate for acute reperfusion therapy with thrombolytics or primary PCI),
- those with ST segment depression or T wave inversion (suspicious for ischemia), and
- those with a so-called non-diagnostic or normal ECG.
A normal ECG does not rule out acute myocardial infarction. Mistakes in interpretation are relatively common, and the failure to identify high risk features has a negative effect on the quality of patient care.[67]
Cardiac markers
Cardiac markers or cardiac enzymes are proteins that leak out of injured myocardial cells through their damaged cell membranes into the bloodstream. Until the 1980s, the enzymes SGOT and LDH were used to assess cardiac injury. Now, the markers most widely used in detection of MI are MB subtype of the enzyme creatine kinase and cardiac troponins T and I as they are more specific for myocardial injury. The cardiac troponins T and I which are released within 4–6 hours of an attack of MI and remain elevated for up to 2 weeks, have nearly complete tissue specificity and are now the preferred markers for asssessing myocardial damage.[68] Heart-type fatty acid binding protein is another marker, used in some home test kits. Elevated troponins in the setting of chest pain may accurately predict a high likelihood of a myocardial infarction in the near future.[69] New markers such as glycogen phosphorylase isoenzyme BB are under investigation.[70]
The diagnosis of myocardial infarction requires two out of three components (history, ECG, and enzymes). When damage to the heart occurs, levels of cardiac markers rise over time, which is why blood tests for them are taken over a 24-hour period. Because these enzyme levels are not elevated immediately following a heart attack, patients presenting with chest pain are generally treated with the assumption that a myocardial infarction has occurred and then evaluated for a more precise diagnosis.[71]
Angiography

In difficult cases or in situations where intervention to restore blood flow is appropriate, coronary angiography can be performed. A catheter is inserted into an artery (usually the femoral artery) and pushed to the vessels supplying the heart. A radio-opaque dye is administered through the catheter and a sequence of x-rays (fluoroscopy) is performed. Obstructed or narrowed arteries can be identified, and angioplasty applied as a therapeutic measure (see below). Angioplasty requires extensive skill, especially in emergency settings. It is performed by a physician trained in interventional cardiology.
Histopathology
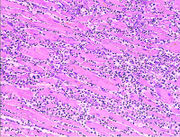
Histopathological examination of the heart may reveal infarction at autopsy. Under the microscope, myocardial infarction presents as a circumscribed area of ischemic, coagulative necrosis (cell death). On gross examination, the infarct is not identifiable within the first 12 hours.[72]

Although earlier changes can be discerned using electron microscopy, one of the earliest changes under a normal microscope are so-called wavy fibers.[73] Subsequently, the myocyte cytoplasm becomes more eosinophilic (pink) and the cells lose their transversal striations, with typical changes and eventually loss of the cell nucleus.[74] The interstitium at the margin of the infarcted area is initially infiltrated with neutrophils, then with lymphocytes and macrophages, who phagocytose ("eat") the myocyte debris. The necrotic area is surrounded and progressively invaded by granulation tissue, which will replace the infarct with a fibrous (collagenous) scar (which are typical steps in wound healing). The interstitial space (the space between cells outside of blood vessels) may be infiltrated with red blood cells.[72]
These features can be recognized in cases where the perfusion was not restored; reperfused infarcts can have other hallmarks, such as contraction band necrosis.[75]
Prevention
The risk of a recurrent myocardial infarction decreases with strict blood pressure management and lifestyle changes, chiefly smoking cessation, regular exercise, a sensible diet for patients with heart disease, and limitation of alcohol intake.
Patients are usually commenced on several long-term medications post-MI, with the aim of preventing secondary cardiovascular events such as further myocardial infarctions, congestive heart failure or cerebrovascular accident (CVA). Unless contraindicated, such medications may include:[76][77]
- Evidence supports the consumption of polyunsaturated fats instead of saturated fats as a measure of decreasing coronary heart disease.[78]
- Antiplatelet drug therapy such as aspirin and/or clopidogrel should be continued to reduce the risk of plaque rupture and recurrent myocardial infarction. Aspirin is first-line, owing to its low cost and comparable efficacy, with clopidogrel reserved for patients intolerant of aspirin. The combination of clopidogrel and aspirin may further reduce risk of cardiovascular events, however the risk of hemorrhage is increased.[79]
- Beta blocker therapy such as metoprolol or carvedilol should be commenced.[80] These have been particularly beneficial in high-risk patients such as those with left ventricular dysfunction and/or continuing cardiac ischaemia.[81] β-Blockers decrease mortality and morbidity. They also improve symptoms of cardiac ischemia in NSTEMI.
- ACE inhibitor therapy should be commenced 24–48 hours post-MI in hemodynamically-stable patients, particularly in patients with a history of MI, diabetes mellitus, hypertension, anterior location of infarct (as assessed by ECG), and/or evidence of left ventricular dysfunction. ACE inhibitors reduce mortality, the development of heart failure, and decrease ventricular remodelling post-MI.[82]
- Statin therapy has been shown to reduce mortality and morbidity post-MI.[83][84] The effects of statins may be more than their LDL lowering effects. The general consensus is that statins have plaque stabilization and multiple other ("pleiotropic") effects that may prevent myocardial infarction in addition to their effects on blood lipids.[85]
- The aldosterone antagonist agent eplerenone has been shown to further reduce risk of cardiovascular death post-MI in patients with heart failure and left ventricular dysfunction, when used in conjunction with standard therapies above.[86] Spironolactone is another option that is sometimes preferable to eplerenone due to cost.
- Omega-3 fatty acids, commonly found in fish, have been shown to reduce mortality post-MI.[87] While the mechanism by which these fatty acids decrease mortality is unknown, it has been postulated that the survival benefit is due to electrical stabilization and the prevention of ventricular fibrillation.[88] However, further studies in a high-risk subset have not shown a clear-cut decrease in potentially fatal arrhythmias due to omega-3 fatty acids.[89][90]
Blood donation may reduce the risk of heart disease for men,[91] but the link has not been firmly established.
Management
An MI is a medical emergency which requires immediate medical attention. Treatment attempts to salvage as much myocardium as possible and to prevent further complications thus the phrase "time is muscle".[92] Oxygen, aspirin, and nitroglycerin are usually administered as soon as possible. Morphine was classically used if nitroglycerin was not effective however it may increase mortality in the setting of NSTEMI.[93] A 2009 and 2010 review of high flow oxygen in myocardial infarction found increased mortality and infarct size, calling into question the recommendation about its routine use.[4][94]
Antiplatelet agents
Aspirin have been shown to markedly reduce mortality.[95] It can be taken quickly (if the person can tolerate aspirin).[96] Aspirin has an antiplatelet effect which inhibits formation of further thrombi (blood clots) that clog arteries. Chewing is the preferred method of administration, so that it can be absorbed quickly. Dissolved soluble preparations or sublingual administration can also be used. U.S. guidelines recommend a dose of 162–325 mg.[97] Australian guidelines recommend a dose of 150–300 mg.[76] Additional antiplatelet agents such as clopidogrel are also used.[97] Some guidelines[98] advise that the dose of Clopidogrel given is dictated by what further treatment is anticipated:
- 300 mg of clopidogrel given orally for patients who are receiving, will receive, thrombolysis
- 600 mg of clopidogrel to be given orally where primary percutaneous coronary intervention (angioplasty) is anticipated.
Nitroglycerin
Glyceryl trinitrate (nitroglycerin) sublingually (under the tongue) or Buccally can be given if available. The Glyceryl Trinitrate acts as a Nitrous oxide donor to smooth muscles cells adjacent to the coronary artery endothelium resulting in increased vasodilation and increased coronary blood flow. Nitroglycerin should not be given if certain inhibitors such as Viagra, Cialis, and Levitra have been taken by the casualty within the previous 12 hours as the combination of the two could cause a serious drop in blood pressure.
Reperfusion
The concept of reperfusion has become so central to the modern treatment of acute myocardial infarction, that we are said to be in the reperfusion era.[99][100] Patients who present with suspected acute myocardial infarction and ST segment elevation (STEMI) or new bundle branch block on the 12 lead ECG are presumed to have an occlusive thrombosis in an epicardial coronary artery. They are therefore candidates for immediate reperfusion, either with thrombolytic therapy, percutaneous coronary intervention (PCI) or when these therapies are unsuccessful, bypass surgery.
Individuals without ST segment elevation are presumed to be experiencing either unstable angina (UA) or non-ST segment elevation myocardial infarction (NSTEMI). They receive many of the same initial therapies and are often stabilized with antiplatelet drugs and anticoagulated. If their condition remains (hemodynamically) stable, they can be offered either late coronary angiography with subsequent restoration of blood flow (revascularization), or non-invasive stress testing to determine if there is significant ischemia that would benefit from revascularization. If hemodynamic instability develops in individuals with NSTEMIs, they may undergo urgent coronary angiography and subsequent revascularization. The use of thrombolytic agents is contraindicated in this patient subset, however.[101]
The basis for this distinction in treatment regimens is that ST segment elevations on an ECG are typically due to complete occlusion of a coronary artery. On the other hand, in NSTEMIs there is typically a sudden narrowing of a coronary artery with preserved (but diminished) flow to the distal myocardium. Anticoagulation and antiplatelet agents are given to prevent the narrowed artery from occluding.
At least 10% of patients with STEMI do not develop myocardial necrosis (as evidenced by a rise in cardiac markers) and subsequent Q waves on EKG after reperfusion therapy. Such a successful restoration of flow to the infarct-related artery during an acute myocardial infarction is known as "aborting" the myocardial infarction. If treated within the hour, about 25% of STEMIs can be aborted.[102]
Rehabilitation
Additional objectives are to prevent life-threatening arrhythmias or conduction disturbances. This requires monitoring in a coronary care unit and protocolised administration of antiarrhythmic agents. Antiarrhythmic agents are typically only given to individuals with life-threatening arrhythmias after a myocardial infarction and not to suppress the ventricular ectopy that is often seen after a myocardial infarction.[103][104][105]
Cardiac rehabilitation aims to optimize function and quality of life in those afflicted with a heart disease. This can be with the help of a physician, or in the form of a cardiac rehabilitation program.[106]
Physical exercise is an important part of rehabilitation after a myocardial infarction, with beneficial effects on cholesterol levels, blood pressure, weight, stress and mood.[106] Some patients become afraid of exercising because it might trigger another infarct.[107] Patients are stimulated to exercise, and should only avoid certain exerting activities. Local authorities may place limitations on driving motorised vehicles.[108] In most cases, the advice is a gradual increase in physical exercise during about 6-8 weeks following an MI.[109] If it doesn't feel too hard for the patient, the advice about exercise is then the same as applies to anyone else to gain health benefits, that is, at least 20-30 minutes of moderate exercise on most days (at least five days per week) to the extent of getting slightly short of breath.[109]
Some people are afraid to have sex after a heart attack. Most people can resume sexual activities after 3 to 4 weeks. The amount of activity needs to be dosed to the patient's possibilities.[110]
Emergency services
When symptoms of myocardial infarction occur, people wait an average of three hours, instead of doing what is recommended: calling for help immediately.[111][112] Acting immediately by calling the emergency services can improve outcomes for two reasons. First and most importantly, the emergency services can immediately save life from ventricular fibrillation, most often primary ventricular fibrillation, which occurs unexpectedly in more than 10% of all infarctions especially during the first hour of symptoms[113] and second, immediate treatment of myocardial infarction can prevent sustained damage to the heart ("time is muscle").[92]
Emergency Medical Services (EMS) Systems vary considerably in their ability to evaluate and treat patients with suspected acute myocardial infarction. Some provide as little as first aid and early defibrillation. Others employ highly trained paramedics with sophisticated technology and advanced protocols.[114] Paramedic services are capable of providing oxygen, IV access, sublingual nitroglycerine, morphine, and aspirin. Some advanced paramedic systems can also perform 12-lead ECGs. If a STEMI is recognized the paramedic may be able to contact the local PCI hospital and alert the emergency room physician, and staff of the suspected AMI. Some Paramedic services are capable of providing thrombolytic therapy in the prehospital setting, allowing reperfusion of the myocardium.[115][116]
With primary PCI emerging as the preferred therapy for ST-segment elevation myocardial infarction, EMS can play a key role in reducing door to balloon intervals (the time from presentation to a hospital ER to the restoration of coronary artery blood flow) by performing a 12-lead ECG in the field and using this information to triage the patient to the most appropriate medical facility.[117][118][119][120] In addition, the 12-lead ECG can be transmitted to the receiving hospital, which enables time saving decisions to be made prior to the arrival of the patient. This may include a "cardiac alert" or "STEMI alert" that calls in off duty personnel in areas where the cardiac cath lab is not staffed 24 hours a day.[121] Even in the absence of a formal alerting program, prehospital 12-lead ECGs are independently associated with reduced door to treatment intervals in the emergency department.[122]
Special cases
- Cocaine
Cocaine associated myocardial infarction should be managed in a manner similar to other patients with acute coronary syndrome except beta blockers should not be used and benzodiazepines should be administered early.[123] The treatment itself may have complications. If attempts to restore the blood flow are initiated after a critical period of only a few hours, the result may be a reperfusion injury instead of amelioration.[124]
- Wilderness setting
In wilderness first aid, a possible heart attack justifies evacuation by the fastest available means, often meaning the initiation of a MEDEVAC. The suspicion or provisional diagnosis of an MI means that it is inappropriate for the patient to walk out of the wilderness setting and will require them to be carried or conveyed in a vehicle. Aspirin, GTN and Oxygen can all be given with relative ease in a wilderness setting and should be administered as soon as possible in suspected cases of MI. Wilderness management of Cardiac Arrest differs slightly from that carried out in an urban setting in that it is generally considered acceptable to terminate a resuscitation attempt after 30 minutes if there have been no change in the patients condition.
- Air travel
Certified personnel traveling by commercial aircraft may be able to assist an MI patient by using the on-board first aid kit, which may contain some cardiac drugs (such as glyceryl trinitrate spray, aspirin, or opioid painkillers), an AED,[125] and oxygen. Pilots may divert the flight to land at a nearby airport. Cardiac monitors are being introduced by some airlines, and they can be used by both on-board and ground-based physicians.[126]
Complications
Complications may occur immediately following the heart attack (in the acute phase), or may need time to develop (a chronic problem). After an infarction, an obvious complication is a second infarction, which may occur in the domain of another atherosclerotic coronary artery, or in the same zone if there are any live cells left in the infarct.
Congestive heart failure
A myocardial infarction may compromise the function of the heart as a pump for the circulation, a state called heart failure. There are different types of heart failure; left- or right-sided (or bilateral) heart failure may occur depending on the affected part of the heart, and it is a low-output type of failure. If one of the heart valves is affected, this may cause dysfunction, such as mitral regurgitation in the case of left-sided coronary occlusion that disrupts the blood supply of the papillary muscles. The incidence of heart failure is particularly high in patients with diabetes and requires special management strategies.[127]
Myocardial rupture
Myocardial rupture is most common three to five days after myocardial infarction, commonly of small degree, but may occur one day to three weeks later. In the modern era of early revascularization and intensive pharmacotherapy as treatment for MI, the incidence of myocardial rupture is about 1% of all MIs.[128] This may occur in the free walls of the ventricles, the septum between them, the papillary muscles, or less commonly the atria. Rupture occurs because of increased pressure against the weakened walls of the heart chambers due to heart muscle that cannot pump blood out effectively. The weakness may also lead to ventricular aneurysm, a localized dilation or ballooning of the heart chamber.
Risk factors for myocardial rupture include completion of infarction (no revascularization performed), female sex, advanced age, and a lack of a previous history of myocardial infarction.[128] In addition, the risk of rupture is higher in individuals who are revascularized with a thrombolytic agent than with PCI.[129][130] The shear stress between the infarcted segment and the surrounding normal myocardium (which may be hypercontractile in the post-infarction period) makes it a nidus for rupture.[131]
Rupture is usually a catastrophic event that may result a life-threatening process known as cardiac tamponade, in which blood accumulates within the pericardium or heart sac, and compresses the heart to the point where it cannot pump effectively. Rupture of the intraventricular septum (the muscle separating the left and right ventricles) causes a ventricular septal defect with shunting of blood through the defect from the left side of the heart to the right side of the heart, which can lead to right ventricular failure as well as pulmonary overcirculation. Rupture of the papillary muscle may also lead to acute mitral regurgitation and subsequent pulmonary edema and possibly even cardiogenic shock.
Arrhythmia

Since the electrical characteristics of the infarcted tissue change (see pathophysiology section), arrhythmias are a frequent complication.[132] The re-entry phenomenon may cause rapid heart rates (ventricular tachycardia and even ventricular fibrillation), and ischemia in the electrical conduction system of the heart may cause a complete heart block (when the impulse from the sinoatrial node, the normal cardiac pacemaker, does not reach the heart chambers).[133][134]
Pericarditis
As a reaction to the damage of the heart muscle, inflammatory cells are attracted. The inflammation may reach out and affect the heart sac. This is called pericarditis. In Dressler's syndrome, this occurs several weeks after the initial event.
Cardiogenic shock
A complication that may occur in the acute setting soon after a myocardial infarction or in the weeks following it is cardiogenic shock. Cardiogenic shock is defined as a hemodynamic state in which the heart cannot produce enough of a cardiac output to supply an adequate amount of oxygenated blood to the tissues of the body.
While the data on performing interventions on individuals with cardiogenic shock is sparse, trial data suggests a long-term mortality benefit in undergoing revascularization if the individual is less than 75 years old and if the onset of the acute myocardial infarction is less than 36 hours and the onset of cardiogenic shock is less than 18 hours.[135] If the patient with cardiogenic shock is not going to be revascularized, aggressive hemodynamic support is warranted, with insertion of an intra-aortic balloon pump if not contraindicated.[135] If diagnostic coronary angiography does not reveal a culprit blockage that is the cause of the cardiogenic shock, the prognosis is poor.[135]
Prognosis
The prognosis post myocardial infarction varies greatly, depending on a person's health, the extent of the heart damage and the treatment given. For the period 2005 - 2008 in the United States the median mortality at 30 days was 16.6% with a range from 10.9% to 24.9% depending on the hospital.[136] Using variables available in the emergency room, people with a higher risk of adverse outcome can be identified. One study found that 0.4% of patients with a low risk profile died after 90 days, whereas in high risk people it was 21.1%.[137]
Some of the more reproduced risk stratifying factors include: age, hemodynamic parameters (such as heart failure, cardiac arrest on admission, systolic blood pressure, or Killip class of two or greater), ST-segment deviation, diabetes, serum creatinine, peripheral vascular disease and elevation of cardiac markers.[137][138][139] Assessment of left ventricular ejection fraction may increase the predictive power.[140] The prognostic importance of Q-waves is debated.[141] Prognosis is significantly worsened if a mechanical complication such as papillary muscle or myocardial free wall rupture occur.[129] Morbidity and mortality from myocardial infarction has improved over the years due to better treatment.[142]
Epidemiology
Myocardial infarction is a common presentation of ischemic heart disease. The WHO estimated in 2002, that 12.6 percent of worldwide deaths were from ischemic heart disease[5] with it the leading cause of death in developed countries, and third to AIDS and lower respiratory infections in developing countries.[143] Worldwide more than 3 million people have STEMIs and 4 million have NSTEMIs a year.[144]
Coronary heart disease is responsible for 1 in 5 deaths in the United States. It is becoming more common in the developing world such that in India, cardiovascular disease (CVD) is the leading cause of death.[145] The deaths due to CVD in India were 32% of all deaths in 2007 and are expected to rise from 1.17 million in 1990 and 1.59 million in 2000 to 2.03 million in 2010.[146] Although a relatively new epidemic in India, it has quickly become a major health issue with deaths due to CVD expected to double during 1985-2015.[147][148] Mortality estimates due to CVD vary widely by state, ranging from 10% in Meghalaya to 49% in Punjab (percentage of all deaths). Punjab (49%), Goa (42%), Tamil Nadu (36%) and Andhra Pradesh (31%) have the highest CVD related mortality estimates.[149] State-wise differences are correlated with prevalence of specific dietary risk factors in the states. Moderate physical exercise is associated with reduced incidence of CVD in India (those who exercise have less than half the risk of those who don't).[147]
Legal implications
At common law, a myocardial infarction is generally a disease, but may sometimes be an injury. This has implications for no-fault insurance schemes such as workers' compensation. A heart attack is generally not covered;[150] however, it may be a work-related injury if it results, for example, from unusual emotional stress or unusual exertion.[151] Additionally, in some jurisdictions, heart attacks suffered by persons in particular occupations such as police officers may be classified as line-of-duty injuries by statute or policy. In some countries or states, a person who has suffered from a myocardial infarction may be prevented from participating in activity that puts other people's lives at risk, for example driving a car or flying an airplane.[108]
Research
Patients who receive stem cell treatment by coronary artery injections of stem cells derived from their own bone marrow after a myocardial infarction (MI) show improvements in left ventricular ejection fraction and end-diastolic volume not seen with placebo. The larger the initial infarct size, the greater the effect of the infusion. Clinical trials of progenitor cell infusion as a treatment approach to ST elevation MI are proceeding.[152]
There are currently 3 biomaterial and tissue engineering approaches for the treatment of MI, but these are in an even earlier stage of medical research, so many questions and issues need to be addressed before they can be applied to patients. The first involves polymeric left ventricular restraints in the prevention of heart failure. The second utilizes in vitro engineered cardiac tissue, which is subsequently implanted in vivo. The final approach entails injecting cells and/or a scaffold into the myocardium to create in situ engineered cardiac tissue.[153]
References
- ↑ 1.0 1.1 1.2 Mallinson, T (2010). "Myocardial Infarction". Focus on First Aid (15): 15. http://www.focusonfirstaid.co.uk/Magazine/issue15/index.aspx. Retrieved 2010-06-08.
- ↑ Kosuge, M; Kimura K, Ishikawa T et al. (March 2006). "Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction". Circulation Journal 70 (3): 222–226. doi:10.1253/circj.70.222. PMID 16501283. http://www.jstage.jst.go.jp/article/circj/70/3/222/_pdf. Retrieved 2008-05-31.
- ↑ Erhardt L, Herlitz J, Bossaert L, et al. (2002). "Task force on the management of chest pain" (PDF). Eur. Heart J. 23 (15): 1153–76. doi:10.1053/euhj.2002.3194. PMID 12206127. http://eurheartj.oxfordjournals.org/cgi/reprint/23/15/1153.
- ↑ 4.0 4.1 "Routine use of oxygen in the treatment of myocardial infarction: systematic review -- Wijesinghe et al. 95 (3): 198 -- Heart". http://heart.bmj.com/cgi/content/full/95/3/198.
- ↑ 5.0 5.1 Robert Beaglehole, et al. (2004) (PDF). The World Health Report 2004 - Changing History. World Health Organization. pp. 120–4. ISBN 92-4-156265-X. http://www.who.int/entity/whr/2004/en/report04_en.pdf.
- ↑ Bax L, Algra A, Mali WP, Edlinger M, Beutler JJ, van der Graaf Y (2008). "Renal function as a risk indicator for cardiovascular events in 3216 patients with manifest arterial disease". Atherosclerosis 200 (1): 184. doi:10.1016/j.atherosclerosis.2007.12.006. PMID 18241872. http://linkinghub.elsevier.com/retrieve/pii/S0021-9150(07)00768-X.
- ↑ Pearte CA, Furberg CD, O'Meara ES, et al. (2006). "Characteristics and baseline clinical predictors of future fatal versus nonfatal coronary heart disease events in older adults: the Cardiovascular Health Study". Circulation 113 (18): 2177–85. doi:10.1161/CIRCULATIONAHA.105.610352. PMID 16651468. http://circ.ahajournals.org/cgi/content/full/113/18/2177.
- ↑ 8.0 8.1 Reznik, AG (2010). "[Morphology of acute myocardial infarction at prenecrotic stage]" (in Russian). Kardiologiia 50 (1): 4–8. PMID 20144151.
- ↑ 9.0 9.1 Moe KT, Wong P (March 2010). "Current trends in diagnostic biomarkers of acute coronary syndrome". Ann. Acad. Med. Singap. 39 (3): 210–5. PMID 20372757. http://www.annals.edu.sg/pdf/39VolNo3Mar2010/V39N3p210.pdf.
- ↑ Thygesen K, Alpert JS, White HD (October 2007). "Universal definition of myocardial infarction". Eur. Heart J. 28 (20): 2525–38. doi:10.1093/eurheartj/ehm355. PMID 17951287. http://eurheartj.oxfordjournals.org/content/28/20/2525.full.
- ↑ National Heart, Lung and Blood Institute. Heart Attack Warning Signs. Retrieved November 22, 2006.
- ↑ Marcus GM, Cohen J, Varosy PD, et al. (2007). "The utility of gestures in patients with chest discomfort". Am. J. Med. 120 (1): 83–9. doi:10.1016/j.amjmed.2006.05.045. PMID 17208083. http://linkinghub.elsevier.com/retrieve/pii/S0002-9343(06)00668-1.
- ↑ Little RA, Frayn KN, Randall PE, et al. (1986). "Plasma catecholamines in the acute phase of the response to myocardial infarction". Arch Emerg Med 3 (1): 20–7. PMID 3524599.
- ↑ 14.0 14.1 Canto JG, Goldberg RJ, Hand MM, et al. (December 2007). "Symptom presentation of women with acute coronary syndromes: myth vs reality". Arch. Intern. Med. 167 (22): 2405–13. doi:10.1001/archinte.167.22.2405. PMID 18071161. http://archinte.ama-assn.org/cgi/pmidlookup?view=long&pmid=18071161.
- ↑ McSweeney JC, Cody M, O'Sullivan P, Elberson K, Moser DK, Garvin BJ (2003). "Women's early warning symptoms of acute myocardial infarction". Circulation 108 (21): 2619–23. doi:10.1161/01.CIR.0000097116.29625.7C. PMID 14597589.
- ↑ D Lee, D Kulick, J Marks. Heart Attack (Myocardial Infarction) by MedicineNet.com . Retrieved November 28, 2006.
- ↑ Kannel WB. (1986). "Silent myocardial ischemia and infarction: insights from the Framingham Study". Cardiol Clin 4 (4): 583–91. PMID 3779719.
- ↑ 18.0 18.1 Davis TM, Fortun P, Mulder J, Davis WA, Bruce DG (2004). "Silent myocardial infarction and its prognosis in a community-based cohort of Type 2 diabetic patients: the Fremantle Diabetes Study". Diabetologia 47 (3): 395–9. doi:10.1007/s00125-004-1344-4. PMID 14963648.
- ↑ Rubin's Pathology — Clinicopathological Foundations of Medicine. Maryland: Lippincott Williams & Wilkins. 2001. pp. 549. ISBN 0-7817-4733-3.
- ↑ Acute Coronary Syndrome. American Heart Association. Retrieved November 25, 2006.
- ↑ Boie ET (2005). "Initial evaluation of chest pain". Emerg. Med. Clin. North Am. 23 (4): 937–57. doi:10.1016/j.emc.2005.07.007. PMID 16199332. http://linkinghub.elsevier.com/retrieve/pii/S0733-8627(05)00059-3.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. "Prediction of coronary heart disease using risk factor categories". Circulation 1998; 97(18): 1837-47. PMID 9603539
- ↑ 23.0 23.1 Saikku P, Leinonen M, Tenkanen L, Linnanmaki E, Ekman MR, Manninen V, Manttari M, Frick MH, Huttunen JK. (1992). "Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study". Ann Intern Med 116 (4): 273–8. PMID 1733381.
- ↑ Andraws R, Berger JS, Brown DL. (2005). "Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials". JAMA 293 (21): 2641–7. doi:10.1001/jama.293.21.2641. PMID 15928286.
- ↑ Muller JE, Stone PH, Turi ZG, et al. (1985). "Circadian variation in the frequency of onset of acute myocardial infarction". N. Engl. J. Med. 313 (21): 1315–22. PMID 2865677.
- ↑ Beamer AD, Lee TH, Cook EF, et al. (1987). "Diagnostic implications for myocardial ischemia of the circadian variation of the onset of chest pain". Am. J. Cardiol. 60 (13): 998–1002. doi:10.1016/0002-9149(87)90340-7. PMID 3673917.
- ↑ Cannon CP, McCabe CH, Stone PH, et al. (1997). "Circadian variation in the onset of unstable angina and non-Q-wave acute myocardial infarction (the TIMI III Registry and TIMI IIIB)". Am. J. Cardiol. 79 (3): 253–8. doi:10.1016/S0002-9149(97)00743-1. PMID 9036740. http://linkinghub.elsevier.com/retrieve/pii/S0002914997007431.
- ↑ Tofler GH, Brezinski D, Schafer AI, et al. (1987). "Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death". N. Engl. J. Med. 316 (24): 1514–8. PMID 3587281.
- ↑ Fantidis P, Perez De Prada T, Fernandez-Ortiz A, et al. (2002). "Morning cortisol production in coronary heart disease patients". Eur. J. Clin. Invest. 32 (5): 304–8. doi:10.1046/j.1365-2362.2002.00988.x. PMID 12027868. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0014-2972&date=2002&volume=32&issue=5&spage=304.
- ↑ Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P Jr, Razak F, Sharma AM, Anand SS; INTERHEART Study Investigators. (2005). "Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study". Lancet 366 (9497): 1640–9. doi:10.1016/S0140-6736(05)67663-5. PMID 16271645.
- ↑ Jensen G, Nyboe J, Appleyard M, Schnohr P. (1991). "Risk factors for acute myocardial infarction in Copenhagen, II: Smoking, alcohol intake, physical activity, obesity, oral contraception, diabetes, lipids, and blood pressure". Eur Heart J 12 (3): 298–308. PMID 2040311.
- ↑ 32.0 32.1 Nyboe J, Jensen G, Appleyard M, Schnohr P. (1989). "Risk factors for acute myocardial infarction in Copenhagen. I: Hereditary, educational and socioeconomic factors. Copenhagen City Heart Study". Eur Heart J 10 (10): 910–6. PMID 2598948.
- ↑ Khader YS, Rice J, John L, Abueita O. (2003). "Oral contraceptives use and the risk of myocardial infarction: a meta-analysis". Contraception 68 (1): 11–7. doi:10.1016/S0010-7824(03)00073-8. PMID 12878281.
- ↑ 34.0 34.1 34.2 34.3 Wilson AM, Ryan MC, Boyle AJ. (2006). "The novel role of C-reactive protein in cardiovascular disease: risk marker or pathogen". Int J Cardiol 106 (3): 291–7. doi:10.1016/j.ijcard.2005.01.068. PMID 16337036.
- ↑ Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association. (2003). "Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association" (PDF). Circulation 107 (3): 499–511. doi:10.1161/01.CIR.0000052939.59093.45. PMID 12551878. http://circ.ahajournals.org/cgi/reprint/107/3/499.pdf.
- ↑ Janket SJ, Baird AE, Chuang SK, Jones JA. (2003). "Meta-analysis of periodontal disease and risk of coronary heart disease and stroke". Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 95 (5): 559–69. doi:10.1038/sj.ebd.6400272. PMID 12738947.
- ↑ Pihlstrom BL, Michalowicz BS, Johnson NW. (2005). "Periodontal diseases". Lancet 366 (9499): 1809–20. doi:10.1016/S0140-6736(05)67728-8. PMID 16298220.
- ↑ Scannapieco FA, Bush RB, Paju S. (2003). "Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review". Ann Periodontol 8 (1): 38–53. doi:10.1902/annals.2003.8.1.38. PMID 14971247.
- ↑ D'Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. (2006). "Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial". Am Heart J 151 (5): 977–84. doi:10.1016/j.ahj.2005.06.018. PMID 16644317.
- ↑ Lourbakos A, Yuan YP, Jenkins AL, Travis J, Andrade-Gordon P, Santulli R, Potempa J, Pike RN. (2001). "Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity" (PDF). Blood 97 (12): 3790–7. doi:10.1182/blood.V97.12.3790. PMID 11389018. http://bloodjournal.hematologylibrary.org/cgi/reprint/97/12/3790.pdf.
- ↑ Qi M, Miyakawa H, Kuramitsu HK. (2003). "Porphyromonas gingivalis induces murine macrophage foam cell formation". Microb Pathog 35 (6): 259–67. doi:10.1016/j.micpath.2003.07.002. PMID 14580389.
- ↑ Spahr A, Klein E, Khuseyinova N, Boeckh C, Muche R, Kunze M, Rothenbacher D, Pezeshki G, Hoffmeister A, Koenig W. (2006). "Periodontal infections and coronary heart disease: role of periodontal bacteria and importance of total pathogen burden in the Coronary Event and Periodontal Disease (CORODONT) study". Arch Intern Med 166 (5): 554–9. doi:10.1001/archinte.166.5.554. PMID 16534043.
- ↑ Warren-Gash C, Smeeth L, Hayward AC (2009). "Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review". Lancet Infect Dis 9 (10): 601–610. doi:10.1016/S1473-3099(09)70233-6. PMID 19778762.
- ↑ Davis TM, Balme M, Jackson D, Stuccio G, Bruce DG (October 2000). "The diagonal ear lobe crease (Frank's sign) is not associated with coronary artery disease or retinopathy in type 2 diabetes: the Fremantle Diabetes Study". Aust N Z J Med 30 (5): 573–7. PMID 11108067.
- ↑ Lichstein E, Chadda KD, Naik D, Gupta PK. (1974). "Diagonal ear-lobe crease: prevalence and implications as a coronary risk factor". N Engl J Med 290 (11): 615–6. PMID 4812503.
- ↑ Miric D, Fabijanic D, Giunio L, Eterovic D, Culic V, Bozic I, Hozo I. (1998). "Dermatological indicators of coronary risk: a case-control study". Int J Cardiol 67 (3): 251–5. doi:10.1016/S0167-5273(98)00313-1. PMID 9894707.
- ↑ Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC (2004). "Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals". JAMA 291 (2): 210–5. doi:10.1001/jama.291.2.210. PMID 14722147. http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=14722147.
- ↑ Detrano R, Guerci AD, Carr JJ, et al. (2008). "Coronary calcium as a predictor of coronary events in four racial or ethnic groups". N. Engl. J. Med. 358 (13): 1336–45. doi:10.1056/NEJMoa072100. PMID 18367736. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=18367736&promo=ONFLNS19.
- ↑ Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD (2005). "Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study". J. Am. Coll. Cardiol. 46 (1): 158–65. doi:10.1016/j.jacc.2005.02.088. PMID 15992651. http://linkinghub.elsevier.com/retrieve/pii/S0735-1097(05)01031-4.
- ↑ http://www.heartscore.org
- ↑ 51.0 51.1 Tsujita K, Kaikita K, Soejima H, Sugiyama S, Ogawa H (April 2010). "[Acute coronary syndrome-initiating factors]" (in Japanese). Nippon Rinsho 68 (4): 607–14. PMID 20387549.
- ↑ 52.0 52.1 Dohi T, Daida H (April 2010). "[Change of concept and pathophysiology in acute coronary syndrome]" (in Japanese). Nippon Rinsho 68 (4): 592–6. PMID 20387546.
- ↑ Woollard KJ, Geissmann F (February 2010). "Monocytes in atherosclerosis: subsets and functions". Nat Rev Cardiol 7 (2): 77–86. doi:10.1038/nrcardio.2009.228. PMID 20065951.
- ↑ Spaan J, Kolyva C, van den Wijngaard J, et al. (September 2008). "Coronary structure and perfusion in health and disease". Philos Transact a Math Phys Eng Sci 366 (1878): 3137–53. doi:10.1098/rsta.2008.0075. PMID 18559321. http://rsta.royalsocietypublishing.org/cgi/pmidlookup?view=long&pmid=18559321.
- ↑ Krijnen PA, Nijmeijer R, Meijer CJ, Visser CA, Hack CE, Niessen HW. (2002). "Apoptosis in myocardial ischaemia and infarction". J Clin Pathol 55 (11): 801–11. doi:10.1136/jcp.55.11.801. PMID 12401816.
- ↑ Myocardial infarction: diagnosis and investigations - GPnotebook, retrieved November 27, 2006.
- ↑ 57.0 57.1 DE Fenton et al. Myocardial infarction - eMedicine, retrieved November 27, 2006.
- ↑ HEART SCAN - Patient information from University College London. Retrieved November 27, 2006.
- ↑ Skoufis E, McGhie AI (1998). "Radionuclide techniques for the assessment of myocardial viability". Tex Heart Inst J 25 (4): 272–9. PMID 9885104.
- ↑ Anonymous (March 1979). "Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature". Circulation 59 (3): 607–9. PMID 761341.
- ↑ Alpert JS, Thygesen K, Antman E, Bassand JP. (2000). "Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction". J Am Coll Cardiol 36 (3): 959–69. doi:10.1016/S0735-1097(00)00804-4. PMID 10987628.
- ↑ 62.0 62.1 S. Garas et al.. Myocardial Infarction. eMedicine. Retrieved November 22, 2006.
- ↑ Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. Harrison's Principles of Internal Medicine. p. 1444. New York: McGraw-Hill, 2005. ISBN 0-07-139140-1.
- ↑ Kasper DL, et al. Harrison's Principles of Internal Medicine. p. 1450.
- ↑ Cannon CP at al. Management of Acute Coronary Syndromes. p. 175. New Jersey: Humana Press, 1999. ISBN 0-89603-552-2.
- ↑ "2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care — Part 8: Stabilization of the Patient With Acute Coronary Syndromes". Circulation 112: IV–89–IV–110. 2005. doi:10.1161/CIRCULATIONAHA.105.166561. http://circ.ahajournals.org/cgi/content/full/112/24_suppl/IV-89.
- ↑ Masoudi FA, Magid DJ, Vinson DR, et al. (October 2006). "Implications of the failure to identify high-risk electrocardiogram findings for the quality of care of patients with acute myocardial infarction: results of the Emergency Department Quality in Myocardial Infarction (EDQMI) study". Circulation 114 (15): 1565–71. doi:10.1161/CIRCULATIONAHA.106.623652. PMID 17015790. http://circ.ahajournals.org/cgi/content/full/114/15/1565.
- ↑ Eisenman A (2006). "Troponin assays for the diagnosis of myocardial infarction and acute coronary syndrome: where do we stand?". Expert Rev Cardiovasc Ther 4 (4): 509–14. doi:10.1586/14779072.4.4.509. PMID 16918269.
- ↑ Aviles RJ, Askari AT, Lindahl B, Wallentin L, Jia G, Ohman EM, Mahaffey KW, Newby LK, Califf RM, Simoons ML, Topol EJ, Berger P, Lauer MS (2002). "Troponin T levels in patients with acute coronary syndromes, with or without renal dysfunction". N Engl J Med 346 (26): 2047–52. doi:10.1056/NEJMoa013456. PMID 12087140.. Summary for laymen
- ↑ Apple FS, Wu AH, Mair J, et al. (2005). "Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome". Clin. Chem. 51 (5): 810–24. doi:10.1373/clinchem.2004.046292. PMID 15774573. http://www.clinchem.org/cgi/content/full/51/5/810.
- ↑ Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE III, Steward DE, Théroux P. (2002). "ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina)" (PDF). J Am Coll Cardiol 40 (7): 1366–74. PMID 12383588. http://www.acc.org/qualityandscience/clinical/guidelines/unstable/incorporated/UA_incorporated.pdf.
- ↑ 72.0 72.1 Rubin's Pathology — Clinicopathological Foundations of Medicine. Maryland: Lippincott Williams & Wilkins. 2001. pp. 546. ISBN 0-7817-4733-3.
- ↑ Eichbaum FW. "'Wavy' myocardial fibers in spontaneous and experimental adrenergic cardiopathies" Cardiology 1975; 60(6): 358–65. PMID 782705
- ↑ S Roy. Myocardial infarction. Retrieved November 28, 2006.
- ↑ Fishbein MC. (1990). "Reperfusion injury". Clin Cardiol 13 (3): 213–7. doi:10.1152/ajpheart.00270.2002 (inactive 2008-06-25). PMID 2182247.
- ↑ 76.0 76.1 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3.
- ↑ Smith A, Aylward P, Campbell T, et al. Therapeutic Guidelines: Cardiovascular, 4th edition. North Melbourne: Therapeutic Guidelines; 2003. ISSN 1327-9513
- ↑ Mozaffarian D, Micha R, Wallace S (2010). "Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials". PLoS Med. 7 (3): e1000252. doi:10.1371/journal.pmed.1000252. PMID 20351774.
- ↑ Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, Diaz R, Commerford PJ, Valentin V, Yusuf S; Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Trial Investigators. (2003). "Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study". Circulation 108 (14): 1682–7. doi:10.1161/01.CIR.0000091201.39590.CB. PMID 14504182.
- ↑ Yusuf S, Peto R, Lewis J, Collins R, Sleight P (1985). "Beta blockade during and after myocardial infarction: an overview of the randomized trials". Prog Cardiovasc Dis 27 (5): 335–71. doi:10.1016/S0033-0620(85)80003-7. PMID 2858114.
- ↑ Dargie HJ. (2001). "Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial". Lancet 357 (9266): 1385–90. doi:10.1016/S0140-6736(00)04560-8. PMID 11356434.
- ↑ Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. (1992). "Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators". N Engl J Med. 327 (10): 669–77. PMID 1386652.
- ↑ Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. (1996). "The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators". N Engl J Med 335 (14): 1001–9. doi:10.1056/NEJM199610033351401. PMID 8801446.
- ↑ Sacks FM, Moye LA, Davis BR, Cole TG, Rouleau JL, Nash DT, Pfeffer MA, Braunwald E. (1998). "Relationship between plasma LDL concentrations during treatment with pravastatin and recurrent coronary events in the Cholesterol and Recurrent Events trial". Circulation 97 (15): 1446–52. PMID 9576424.
- ↑ Ray KK, Cannon CP (2005). "The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes". J. Am. Coll. Cardiol. 46 (8): 1425–33. doi:10.1016/j.jacc.2005.05.086. PMID 16226165. http://linkinghub.elsevier.com/retrieve/pii/S0735-1097(05)01773-0.
- ↑ Keating G, Plosker G (2004). "Eplerenone: a review of its use in left ventricular systolic dysfunction and heart failure after acute myocardial infarction". Drugs 64 (23): 2689–707. doi:10.1157/13089615. PMID 15537370.
- ↑ "Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico". Lancet 354 (9177): 447–55. 2001. doi:10.1016/S0140-6736(99)07072-5. PMID 10465168.
- ↑ Leaf A, Albert C, Josephson M, Steinhaus D, Kluger J, Kang J, Cox B, Zhang H, Schoenfeld D (2005). "Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake". Circulation 112 (18): 2762–8. doi:10.1161/CIRCULATIONAHA.105.549527. PMID 16267249. http://circ.ahajournals.org/cgi/content/full/112/18/2762.
- ↑ Brouwer IA, Zock PL, Camm AJ, Bocker D, Hauer RN, Wever EF, Dullemeijer C, Ronden JE, Katan MB, Lubinski A, Buschler H, Schouten EG; SOFA Study Group. (2006). "Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial". JAMA 295 (22): 2613–9. doi:10.1001/jama.295.22.2613. PMID 16772624.
- ↑ Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, McClelland J, Cook J, MacMurdy K, Swenson R, Connor SL, Gerhard G, Kraemer DF, Oseran D, Marchant C, Calhoun D, Shnider R, McAnulty J. (2005). "Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial". JAMA 293 (23): 2284–91. doi:10.1001/jama.293.23.2884. PMID 15956633.
- ↑ Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT (Mar 1997). "Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland". BMJ 314 (7083): 793–4. PMID 9080998. PMC 2126176. http://bmj.bmjjournals.com/cgi/content/full/314/7083/793.
- ↑ 92.0 92.1 TIME IS MUSCLE TIME WASTED IS MUSCLE LOST. Early Heart Attack Care, St. Agnes Healthcare. Retrieved November 29, 2006.
- ↑ Meine TJ, Roe MT, Chen AY, et al. (2005). "Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative". Am Heart J 149 (6): 1043–9. doi:10.1016/j.ahj.2005.02.010. PMID 15976786. http://linkinghub.elsevier.com/retrieve/pii/S0002870305001493.
- ↑ Cabello JB, Burls A, Emparanza JI, Bayliss S, Quinn T (2010). "Oxygen therapy for acute myocardial infarction". Cochrane Database Syst Rev 6: CD007160. doi:10.1002/14651858.CD007160.pub2. PMID 20556775.
- ↑ ISIS-2 Collaborative group (1988). "Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2". Lancet 2 (8607): 349–60. PMID 2899772.
- ↑ Brown AL, Mann NC, Daya M, Goldberg R, Meischke H, Taylor J, Smith K, Osganian S, Cooper L. (2000). "Demographic, belief, and situational factors influencing the decision to utilize emergency medical services among chest pain patients. Rapid Early Action for Coronary Treatment (REACT) study". Circulation 102 (2): 173–8. PMID 10889127.
- ↑ 97.0 97.1 Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr (2004). "ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction)". J Am Coll Cardiol 44 (3): 671–719. doi:10.1016/j.jacc.2004.07.002. PMID 15358045. http://www.acc.org/qualityandscience/clinical/guidelines/stemi/Guideline1/index.htm.
- ↑ http://www2.warwick.ac.uk/fac/med/research/hsri/emergencycare/prehospitalcare/jrcalcstakeholderwebsite/notices-copy/
- ↑ Lee KL, Woodlief LH, Topol EJ, et al. "Predictors of 30-Day Mortality in the Era of Reperfusion for Acute Myocardial Infarction." Circulation 1995; 91: 1659-1668. PMID 7882472
- ↑ Stone GW, Grines CL, Browne KF, et al. "Predictors of in-hospital and 6-month outcome after acute myocardial infarction in the reperfusion era: the Primary Angioplasty in Myocardial Infarction (PAMI) trail." J Am Coll Cardiol 1995; 25: 370-377. PMID 14645641
- ↑ "Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group." Lancet 1994; 343(8893): 311-22. PMID 7905143
- ↑ Verheugt FW, Gersh BJ, Armstrong PW. "Aborted myocardial infarction: a new target for reperfusion therapy." Eur Heart J 2006; 27(8): 901-4. PMID 16543251
- ↑ Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, et al. (1991). "Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial". N Engl J Med 324 (12): 781–8. PMID 1900101.
- ↑ Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP. (1996). "Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol". Lancet 348 (9019): 7–12. doi:10.1016/S0140-6736(96)02149-6. PMID 8691967.
- ↑ Julian DG, Camm AJ, Frangin G, Janse MJ, Munoz A, Schwartz PJ, Simon P. (1997). "Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators". Lancet 349 (9053): 667–74. doi:10.1016/S0140-6736(96)09145-3. PMID 9078197.
- ↑ 106.0 106.1 Life after a Heart Attack. U.S. National Heart, Lung and Blood Institute. Retrieved December 2, 2006.
- ↑ Trisha Macnair. Recovering after a heart attack. BBC, December 2005. Retrieved December 2, 2006.
- ↑ 108.0 108.1 "Classification of Drivers' Licenses Regulations". Nova Scotia Registry of Regulations. May 24, 2000. http://www.gov.ns.ca/just/regulations/regs/mvclasdl.htm. Retrieved April 22, 2007.
- ↑ 109.0 109.1 Patient UK > After a Myocardial Infarction Reviewed: 19 May 2010
- ↑ "Heart Attack: Getting Back Into Your Life After a Heart Attack". American Academy of Family Physicians, updated March 2005. Retrieved December 4, 2006.
- ↑ Heart attack first aid. MedlinePlus. Retrieved December 3, 2006.
- ↑ Act In Time to Heart Attack Signs - NHLBI. Retrieved December 13, 2006.
- ↑ Gheeraert, Peter. [http:// www.primaryventricularfibrillation.com/phdthesis.pdf "Ventricular fibrillation during acute myocardial infarction"]. http:// www.primaryventricularfibrillation.com/phdthesis.pdf. Retrieved 13 August 2010.
- ↑ Antman EM, Anbe DT, Armstrong PW, et al. (August 2004). "ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction)". J. Am. Coll. Cardiol. 44 (3): 671–719. doi:10.1016/j.jacc.2004.07.002. PMID 15358045.
- ↑ Morrow DA, Antman EM, Sayah A, et al. (July 2002). "Evaluation of the time saved by prehospital initiation of reteplase for ST-elevation myocardial infarction: results of The Early Retavase-Thrombolysis in Myocardial Infarction (ER-TIMI) 19 trial". J. Am. Coll. Cardiol. 40 (1): 71–7. doi:10.1016/S0735-1097(02)01936-8. PMID 12103258. http://linkinghub.elsevier.com/retrieve/pii/S0735109702019368.
- ↑ Morrison LJ, Verbeek PR, McDonald AC, Sawadsky BV, Cook DJ. (2000). "Mortality and prehospital thrombolysis for acute myocardial infarction: A meta-analysis" (PDF). JAMA 283 (20): 2686–92. doi:10.1001/jama.283.20.2686. PMID 10819952. http://jama.ama-assn.org/cgi/reprint/283/20/2686.pdf?ijkey=c72b289825a3fd6ace7545ef61cd70936485e7e1.
- ↑ Rokos IC, Larson DM, Henry TD, et al. (2006). "Rationale for establishing regional ST-elevation myocardial infarction receiving center (SRC) networks". Am. Heart J. 152 (4): 661–7. doi:10.1016/j.ahj.2006.06.001. PMID 16996830.
- ↑ Moyer P, Feldman J, Levine J, et al. (June 2004). "Implications of the Mechanical (PCI) vs Thrombolytic Controversy for ST Segment Elevation Myocardial Infarction on the Organization of Emergency Medical Services: The Boston EMS Experience". Crit Pathw Cardiol 3 (2): 53–61. doi:10.1097/01.hpc.0000128714.35330.6d. PMID 18340140.
- ↑ Terkelsen CJ, Lassen JF, Nørgaard BL, et al. (April 2005). "Reduction of treatment delay in patients with ST-elevation myocardial infarction: impact of pre-hospital diagnosis and direct referral to primary percutanous coronary intervention". Eur. Heart J. 26 (8): 770–7. doi:10.1093/eurheartj/ehi100. PMID 15684279.T
- ↑ Henry TD, Atkins JM, Cunningham MS, et al. (April 2006). "ST-segment elevation myocardial infarction: recommendations on triage of patients to heart attack centers: is it time for a national policy for the treatment of ST-segment elevation myocardial infarction?". J. Am. Coll. Cardiol. 47 (7): 1339–45. doi:10.1016/j.jacc.2005.05.101. PMID 16580518.
- ↑ Rokos I. and Bouthillet T., "The emergency medical systems-to-balloon (E2B) challenge: building on the foundations of the D2B Alliance," STEMI Systems, Issue Two, May 2007. Accessed June 16, 2007.
- ↑ Cannon, Christopher (1999). Management of acute coronary syndromes. Totowa, NJ: Humana Press. ISBN 0-89603-552-2.
- ↑ McCord J, Jneid H, Hollander JE, et al. (April 2008). "Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology". Circulation 117 (14): 1897–907. doi:10.1161/CIRCULATIONAHA.107.188950. PMID 18347214.
- ↑ Faxon DP. "Coronary interventions and their impact on post myocardial infarction survival." Clin Cardiol 2005; 28(11 Suppl 1):I38-44. PMID 16450811
- ↑ Youngwith, Janice (2008-02-06). "Saving hearts in the air". Dailyherald.com. http://www.dailyherald.com/special/americanheartmonth/2008/index.asp?id=11. Retrieved 2008-06-12.
- ↑ Dowdall N. "'Is there a doctor on the aircraft?' Top 10 in-flight medical emergencies." BMJ 2000; 321(7272):1336-7. PMID 11090520. Full text at PMC: 1119071
- ↑ Canto JG, Shlipak MG, Rogers WJ, Malmgren JA, Frederick PD, Lambrew CT, Ornato JP, Barron HV, Kiefe CI. (2000). "Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain". JAMA 283 (24): 3223–9. doi:10.1001/jama.283.24.3223. PMID 10866870.
- ↑ 128.0 128.1 Yip HK, Wu CJ, Chang HW, Wang CP, Cheng CI, Chua S, Chen MC. (2003). "Cardiac rupture complicating acute myocardial infarction in the direct percutaneous coronary intervention reperfusion era" (PDF). Chest 124 (2): 565–71. doi:10.1378/chest.124.2.565. PMID 12907544. http://www.chestjournal.org/cgi/reprint/124/2/565.pdf.
- ↑ 129.0 129.1 Becker RC, Gore JM, Lambrew C, Weaver WD, Rubison RM, French WJ, Tiefenbrunn AJ, Bowlby LJ, Rogers WJ. (1996). "A composite view of cardiac rupture in the United States National Registry of Myocardial Infarction". J Am Coll Cardiol 27 (6): 1321–6. doi:10.1016/0735-1097(96)00008-3. PMID 8626938.
- ↑ Moreno R, Lopez-Sendon J, Garcia E, Perez de Isla L, Lopez de Sa E, Ortega A, Moreno M, Rubio R, Soriano J, Abeytua M, Garcia-Fernandez MA. (2002). "Primary angioplasty reduces the risk of left ventricular free wall rupture compared with thrombolysis in patients with acute myocardial infarction". J Am Coll Cardiol 39 (4): 598–603. doi:10.1016/S0735-1097(01)01796-X. PMID 11849857.
- ↑ Shin P, Sakurai M, Minamino T, Onishi S, Kitamura H. (1983). "Postinfarction cardiac rupture. A pathogenetic consideration in eight cases". Acta Pathol Jpn 33 (5): 881–93. PMID 6650169.
- ↑ Podrid, Philip J.; Peter R. Kowey (2001). Cardiac Arrhythmia: Mechanisms, Diagnosis, and Management. Lippincott Williams & Wilkins. ISBN 0781724864.
- ↑ Sung, Ruey J.; Michael R. Lauer (2000). Fundamental Approaches to the Management of Cardiac Arrhythmias. Springer. ISBN 0792365593.
- ↑ Josephson, Mark E. (2002). Clinical Cardiac Electrophysiology: Techniques and Interpretations. Lippincott Williams & Wilkins. ISBN 0683306936.
- ↑ 135.0 135.1 135.2 Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH. (1999). "Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock". N Engl J Med 341 (9): 625–34. doi:10.1056/NEJM199908263410901. PMID 10460813.
- ↑ Krumholz H et al (2009). "Patterns of hospital performance in acute myocardial infarction and heart failure - 30-day mortality and readmission". Circulation: Cardiovascular Quality and Outcomes 2 (5): 407. doi:10.1161/CIRCOUTCOMES.109.883256. PMID 20031870. http://circoutcomes.ahajournals.org/cgi/content/abstract/CIRCOUTCOMES.109.883256v1.
- ↑ 137.0 137.1 López de Sá E, López-Sendón J, Anguera I, Bethencourt A, Bosch X (November 2002). "Prognostic value of clinical variables at presentation in patients with non-ST-segment elevation acute coronary syndromes: results of the Proyecto de Estudio del Pronóstico de la Angina (PEPA)". Medicine (Baltimore) 81 (6): 434–42. doi:10.1097/00005792-200211000-00004. PMID 12441900.
- ↑ Fox KA, Dabbous OH, Goldberg RJ, et al. (November 2006). "Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE)". BMJ 333 (7578): 1091. doi:10.1136/bmj.38985.646481.55. PMID 17032691. PMC 1661748. http://www.bmj.com/cgi/content/full/333/7578/1091.
- ↑ Weir RA, McMurray JJ, Velazquez EJ. (2006). "Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance". Am J Cardiol 97 (10A): 13F–25F. doi:10.1016/j.amjcard.2006.03.005. PMID 16698331.
- ↑ Bosch X, Theroux P. (2005). "Left ventricular ejection fraction to predict early mortality in patients with non-ST-segment elevation acute coronary syndromes". Am Heart J 150 (2): 215–20. doi:10.1016/j.ahj.2004.09.027. PMID 16086920.
- ↑ Nicod P, Gilpin E, Dittrich H, Polikar R, Hjalmarson A, Blacky A, Henning H, Ross J (1989). "Short- and long-term clinical outcome after Q wave and non-Q wave myocardial infarction in a large patient population". Circulation 79 (3): 528–36. PMID 2645061.
- ↑ Liew R, Sulfi S, Ranjadayalan K, Cooper J, Timmis AD. (2006). "Declining case fatality rates for acute myocardial infarction in South Asian and white patients in the past 15 years". Heart 92 (8): 1030–4. doi:10.1136/hrt.2005.078634. PMID 16387823.
- ↑ "Cause of Death — UC Atlas of Global Inequality". Center for Global, International and Regional Studies (CGIRS) at the University of California Santa Cruz. http://ucatlas.ucsc.edu/cause.php. Retrieved December 7, 2006.
- ↑ White HD, Chew DP (August 2008). "Acute myocardial infarction". Lancet 372 (9638): 570–84. doi:10.1016/S0140-6736(08)61237-4. PMID 18707987.
- ↑ Mukherjee AK. (1995). "Prediction of coronary heart disease using risk factor categories". J Indian Med Assoc 93 (8): 312–5. PMID 8713248.
- ↑ Ghaffar A, Reddy KS and Singhi M (2004). "Burden of non-communicable diseases in South Asia" (PDF). BMJ 328 (7443): 807–810. doi:10.1136/bmj.328.7443.807. PMID 15070638. PMC 383378. http://www.bmj.com/cgi/reprint/328/7443/807.pdf.
- ↑ 147.0 147.1 Rastogi T, Vaz M, Spiegelman D, Reddy KS, Bharathi AV, Stampfer MJ, Willett WC and Ascherio1 A (2004). "Physical activity and risk of coronary heart disease in India" (PDF). Int. J. Epidemiol 33 (4): 1–9. doi:10.1093/ije/dyh042. PMID 15044412. http://ije.oxfordjournals.org/cgi/reprint/33/4/759.pdf.
- ↑ Gupta R. (2007). "Escalating Coronary Heart Disease and Risk Factors in South Asians" (PDF). Indian Heart Journal: 214–17. http://indianheartjournal.com/editorial007.pdf.
- ↑ Gupta R, Misra A, Pais P, Rastogi P and Gupta VP. (2006). "Correlation of regional cardiovascular disease mortality in India with lifestyle and nutritional factors" (PDF). International Journal of Cardiology 108 (3): 291–300. doi:10.1016/j.ijcard.2005.05.044. PMID 15978684. http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6T16-4GFV5CY-4-3&_cdi=4882&_user=209690&_orig=search&_coverDate=04%2F14%2F2006&_sk=998919996&view=c&wchp=dGLzVlz-zSkzV&md5=050edf99a813f475b985c8d590c86228&ie=/sdarticle.pdf.
- ↑ Workers' Compensation FAQ. Prairie View A&M University. Retrieved November 22, 2006.
- ↑ SIGNIFICANT DECISIONS Subject Index. Board of Industrial Insurance Appeals. Retrieved November 22, 2006.
- ↑ Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM; REPAIR-AMI Investigators (2006). "Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction". N Engl J Med 355 (12): 1210–21. doi:10.1056/NEJMoa060186. PMID 16990384.
- ↑ Christman KL, Lee RJ. "Biomaterials for the Treatment of Myocardial Infarction". J Am Coll Cardiol 2006; 48(5): 907-13. PMID 16949479
External links
- American Heart Association's Heart Attack web site - Information and resources for preventing, recognizing and treating heart attack.
- Heartscore: an interactive tool for prediction and risk management of stroke and heart attack (EU)
- Heart Attack - overview of resources from MedlinePlus.
- Risk Assessment Tool for Estimating 10-year Risk of Having a Heart Attack - based on information of the Framingham Heart Study, from the United States National Heart, Lung and Blood Institute
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||